Research of Ishihara Lab (in English)
Mitochondrial dynamics: Molecular mechanism and physiological roles of fusion and fission in mammals
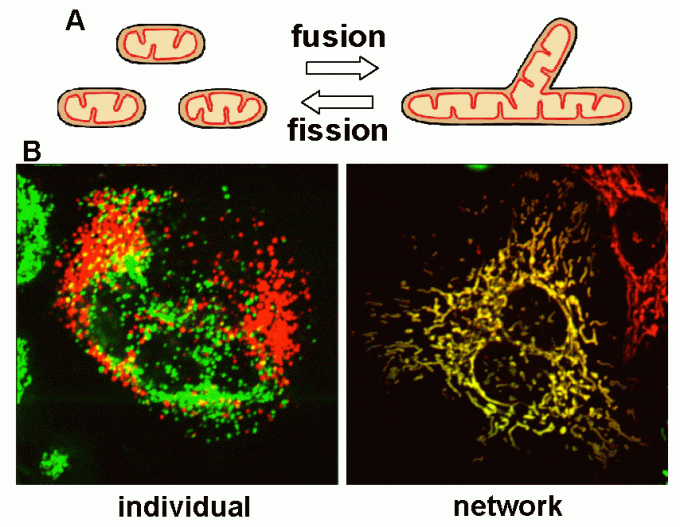
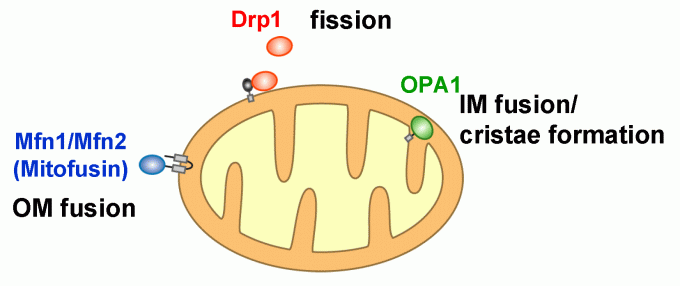
Mitochondria, double membrane-bound organelles with tubular network structures, are essential for oxidative ATP production and play pivotal roles in regulating calcium homeostasis, ROS production and apoptosis. Mitochondria dynamically change their morphology by frequent fusion and fission (Fig 1), and three types of high molecular-weight GTPase proteins have been identified as core components of the fusion and fission machineries (Fig 2).
We have been analyzed their molecular mechanism and the physiological roles in mammals, and found that the regulation of mitochondrial dynamics coupled with a quality control system is essential for cellular homeostasis, development and tissue differentiation.
Fig 1
Fig 2
Mitochondrial fusion regulated by two mitofusin (Mfn) isoforms in mammalian cells
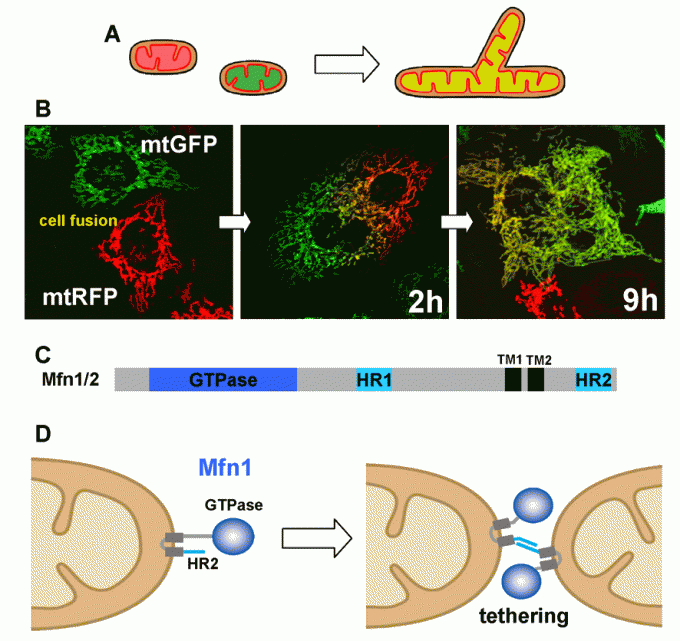
To analyze mitochondrial fusion in living mammalian cells, we have established assay systems. In the fused cells, two fluorescent reporters in the mitochondrial matrix (GFP and RFP, respectively) are mixed in a time-dependent manner, indicating that both outer and inner membranes have fused to facilitate content exchange (Ishihara BBRC 2003, Eura JB 2003, etc) (Fig. 3A).
Mfn proteins contain the N-terminal GTPase domain and are anchored to the outer membrane by two C-terminal transmembrane segments (Fig. 3B). Both Mfn isoforms, Mfn1 and Mfn2, are required for active mitochondrial fusion, and Mfn1 proteins on opposing mitochondria form trans-oligomeric complexes for tethering the outer membranes in a GTP dependent manner (Ishihara JCS 2004, Eura JCS 2006) (Fig. 3C).
Fig 3
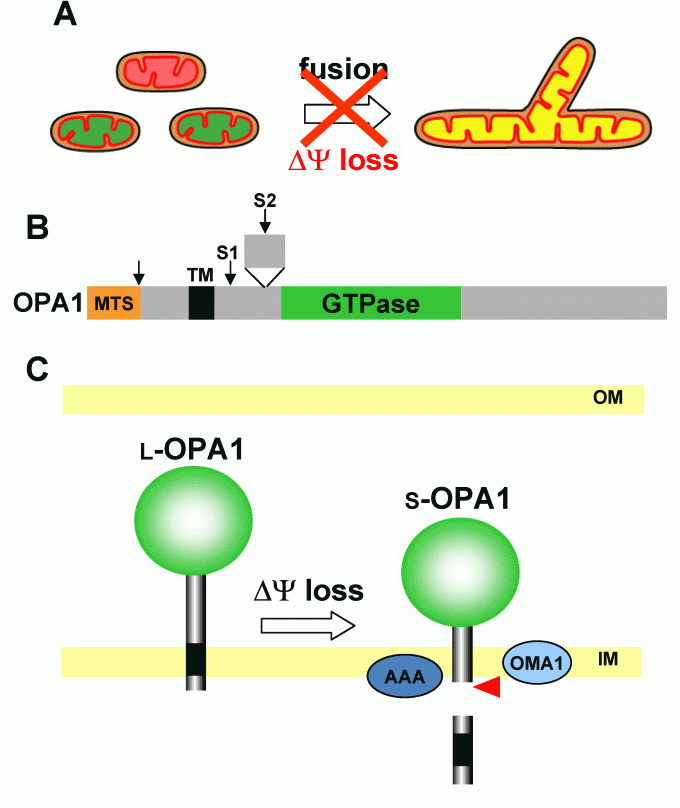
Regulation of mitochondrial inner membrane fusion by OPA1
We found that disruption of membrane potential caused severe inhibition of mitochondrial fusion, thus membrane potential is required for fusion (Fig. 1B right, Fig 4A), and we further showed that OPA1 is involved in the regulation of mitochondrial inner membrane fusion (Ishihara BBRC 2003, Ishihara EMBO J 2006, etc). OPA1 is anchored to the inner membrane by the N-terminal transmembrane domain as large molecular size (L-OPA1). L-OPA1 is proteolytically cleaved to form small molecular size (S-OPA1) molecules, which caused repression of the mitochondrial fusion (Fig. 4 BC).
Fig 4
Regulation of mitochondrial fission by Drp1 modifications
Drp1 plays an essential role in mitochondrial fission. We first found that Drp1 is regulated by phosphorylation (Taguchi JBC 2007). During cell cycle progression, Ser616 in human Drp1 (Ser585 in rat Drp1) is specifically phosphorylated by MPF (Cdk1/cyclinB), which promotes mitochondrial fission in the early mitotic phase. Now it was well known that various post-translational modifications regulates Drp1 function. Phosphorylation at Ser637 in the GTPase effecter domain of human Drp1 (Ser656 in rat Drp1) by PKA affects Drp1 GTPase activity, and regulates mitochondrial fission. Other post-translational modifications such as ubiquitination, sumolylation and nitrosylations are also concerned in the regulation of Drp1.
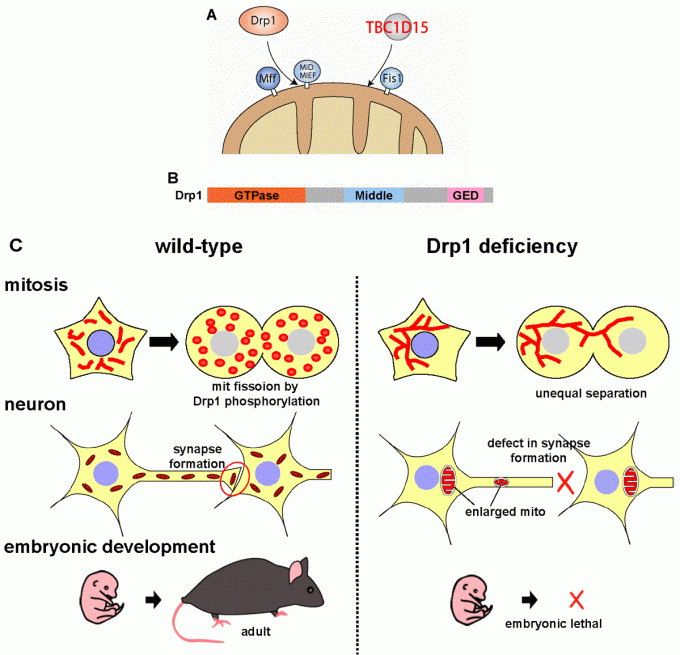
Mitochondrial recruitment and physiological roles of Drp1
Drp1 is recruited from the cytoplasm to the mitochondrial fission sites. In yeast, a C-tail anchored mitochondrial outer membrane protein Fis1 has essential roles in Drp1 recruitment in conjunction with the adaptor proteins, however, Fis1 is dispensable for Drp1 recruitment in mammals (Fig. 5AB). Instead, other factors on the mitochondrial outer membrane, Mff, MiD49, MiD51, are involved in Drp1 recruitment. On the other hands, we recently found that Fis1 in mammalian cell acts as a mitochondrial recruitment factor for TBC1D15, a Rab GTPase effecter, that is involved in regulation of mitochondrial morphology (Onoue JCS 2013).
To elucidate the physiologic roles of mitochondrial fission in vivo, we generated tissue-specific Drp1 KO mice (Ishihara Nat Cell Biol 2009) (Fig 5C). Drp1-KO mice die in embryonic stage with developmental abnormalities. Analysis of neuron-specific Drp1 KO mice showed that Drp1-deficiency causes the abnormal distribution of enlarged mitochondria in extremely polarized cells such as neurites.
Fig 5
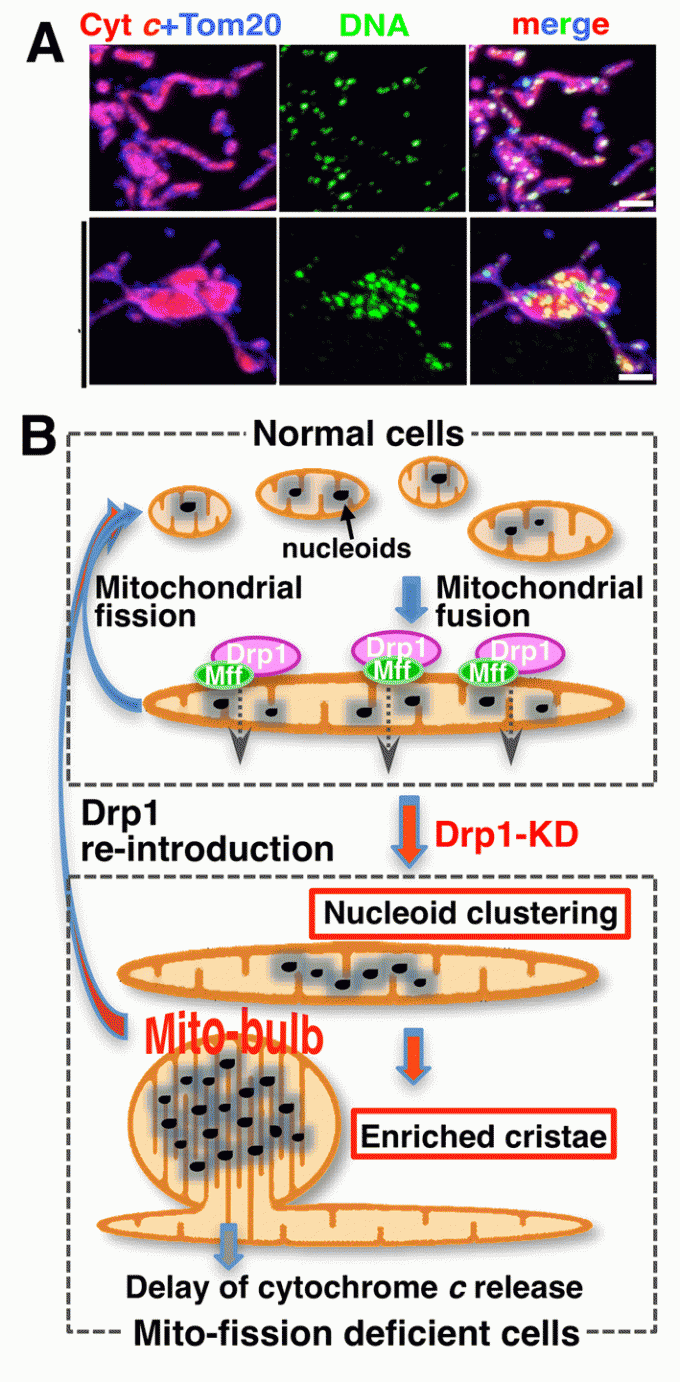
New role of mitochondrial dynamics: Regulation of mtDNA nucleoid morphology
Mammalian cells typically contain thousands of copies of mitochondrial DNA (mtDNA) assembled into hundreds of nucleoids. Recently we found that nucleoid morphology/dynamic are regulated by balanced fusion and fission (Ban-Ishihara PNAS 2013) (Fig 6). In healthy cells, mitochondrial fission often occurred adjacent to nucleoids. In Drp1-deficient cells, nucleoids were highly enlarged by their clustering within hyperfused mitochondria. Thus, mitochondrial fission adjacent to nucleoids should prevent clustering by maintaining small and fragmented nucleoids.
The enhanced clustering of nucleoids resulted in the formation of highly stacked cristae structures in enlarged bulb-like mitochondria (mito-bulbs). Formation of highly stacked cristae suppressed signaling of apoptotic stimuli. Thus, mitochondrial dynamics by fission and fusion play a critical role in controlling mitochondrial nucleoid structures, contributing to cristae reformation and the proapoptotic status of mitochondria (Fig 6B).
Fig 6
Now we are further analyzing the molecular details of mitochondrial double membrane fusion and fission using biochemical and cell biological methods, and their physiological significance in development (Nat Cell Biol 2009, Curr Biol 2014 etc), regulation and quality control (EMBO J 2006, JBC 2011, Nat Cell Biol 2017, etc), and inheritance of mtDNA (PNAS 2013, Mol Cell Biol 2015, etc).